Online programme on “International Regulatory Requirements on Clinical Trials and Data Management”
Trinity College for Women, Namakkal, On behalf of the Naan Mudhalvan, an online programme was held on October 17, 2023.
Dr. Kuriakose Joy, Medical Writer, Spinos, Coimbatore, spoke on the topic of “International Regulatory Requirements on Clinical Trials and Data Management.”
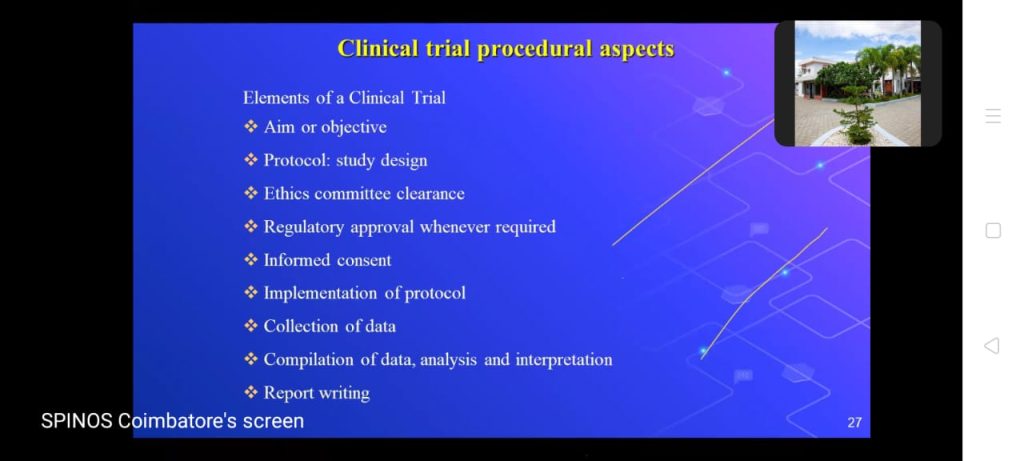
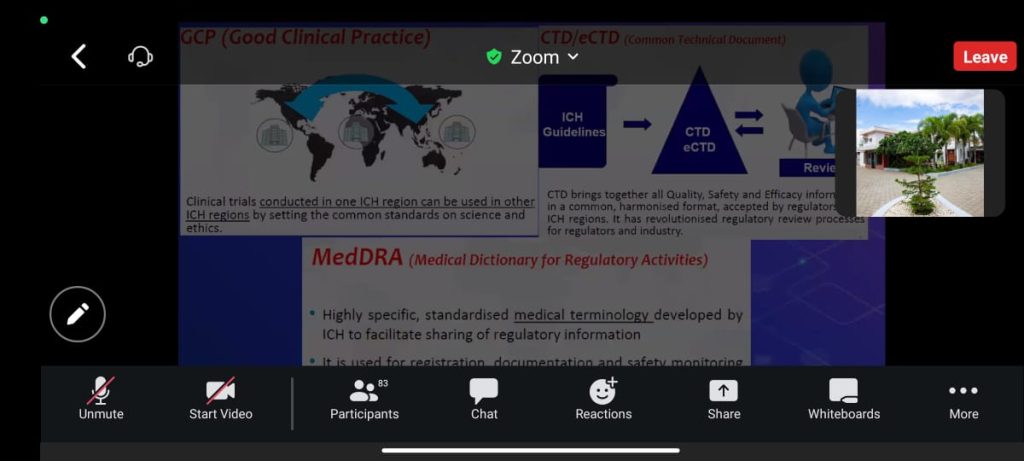
Drug firms must ensure that clinical data generation is reliable. CDM (Clinical Data Management) is a significant phase in clinical research, he denoted.
He also said, it generates high-quality, reliable, and statistically sound data from clinical trials. Also, he pointed out the computer applications, cleaning, and management of clinical data in that CDM.
This programme was arranged by Dr. B. Vishnupriya, Coordinator, Mrs. D. Prabha and Mrs. K. Gowrimala, Adjacents, and the members of Naan Mudhalvan, Mrs. K. Padmavathi, Mrs. S. Madhukaraveni, Ms. P. Gowthami, Dr. S. Anidha, Mrs. V. Gayathri, Ms. L. Keerthi, Ms. P. Sri Renugadevi, and Ms. K.P. Theepika.